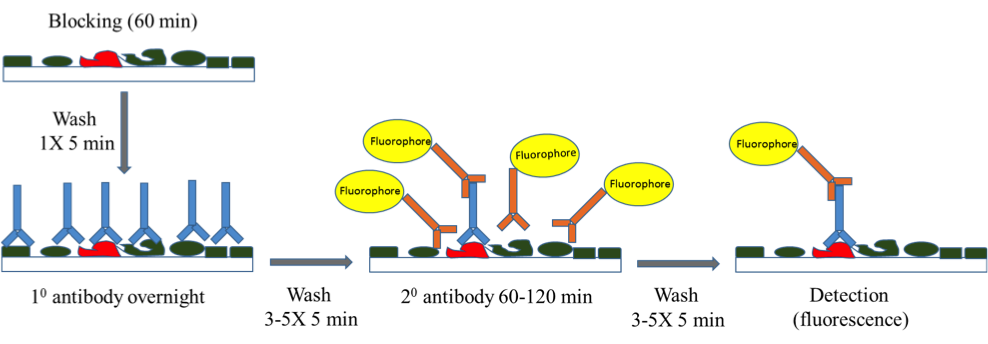
Introduction
Western blot is a widely used method to detect the presence of protein(s) of interest in a given sample and/or detect the post-translational modification such as phosphorylation. The proteins are run on the SDS-PAGE and then transferred onto the surface of the membrane, providing access to immunodetection reagents. Immunodetection then proceeds as described in Fig.1.
Fig. 1A
Fig. 1B
Fig. 1. Workflow of a typical immunodetection protocol by western blot.
After nonspecific binding sites are blocked, usually by adding dry milk or Bovine Serum Albumin (BSA) to a wash buffer saline solution that contains a mild detergent, the membrane is probed with the primary antibody and then washed 3-5 times. The antibody-antigen complexes are then detected by a secondary antibody conjugated to a fluorophore (A) or an enzyme such as Horseradish Peroxidase (HRP) that converts a substrate into a light-emitting molecule (B). After 3-5 washes, the resulting complex is detected by fluorescence or chemiluminescence respectively.
BlotCyclerTM automates all the steps in the protocol starting from blocking and up to the washing steps after the incubation with the secondary antibodies. Compared to the traditionally used manual protocols, BlotCyclerTM provides the end-user with an opportunity to program the precise duration of all the steps as well as the number of washes. Furthermore, the instrument’s shaking and fluidic systems ensure consistent and reproducible dispensing, mixing, and distribution of the reagents throughout the process and provide with an option to recycle primary antibodies. Finally, BlotCyclerTM enables a simultaneous processing of up to twelve blots, while using up to six different primary and secondary antibodies and two separate protocols. Taken together, BlotCyclerTM provides substantial savings in time, money, and increased throughput while enabling the standardization of the western blot procedure that is difficult to achieve using the manual processing.
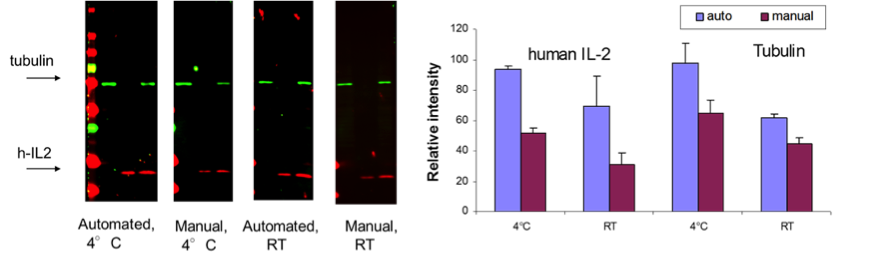
BlotCyclerTM automated western blot processing provides increased sensitivity over the manual procedure
It is a common practice to perform the incubation with primary antibody at 40C overnight and conduct the rest of the steps at room temperature during the manual procedure, as a convenience of not having to frequently go to the 40C room for solution changes. However this has been shown to eliminate advantages of incubation at low temperature on background and signal intensity. To further dissect the role of automation and incubation temperature in the increased signal, the manual and automatic processing were carried out entirely at 4°C or RT (Fig. 2). The results indicate that while both automation and 4°C alone increased the signal, the effect was most pronounced in combination of the two. Therefore, BlotCyclerTM offers the distinct advantage in improved sensitivity of western blotting procedure while eliminating the drudgery associated with the manual processing.
Fig. 2A Fig. 2B
Fig. 2. Automated western blot processing by BlotCyclerTM increased the signal as compared to the manual procedure.
(A) Images of the blots processed as indicated. In each blot, the first lane contained a HeLa whole cell lysate, the second lane contained a recombinant Human IL-2, and the third lane a mixture of HeLa whole cell lysate and h-IL2. Following the SDS-PAGE electrophoresis, proteins were transferred from the gel onto the Nitrocellulose membrane. Membranes were blocked for 30 min at RT or for 90 min at 4ºC using MB-070. Rabbit anti-IL2 and mouse anti-α-tubulin primary antibodies were incubated for 90 min at RT or for 18 hours at 4°C. DyLight™ 549 conjugated anti-mouse IgG and DyLight™ 649 conjugated anti-rabbit IgG secondary antibodies were incubated for 30 min at RT or 90 min at 4°C. Data for each DyLight™ fluorophore were collected independently at excitation/emission wavelengths: 530nm/605nm for the DyLight™ 549 and 625nm/695nm for DyLight™ 649. The fluorescent intensity between different blots was normalized using MW standard as shown in (B).
BlotcyclerTM system’s western blot processing is highly reproducible
To determine if the automated western blot processing results in highly reproducible signal, five identical blots were subjected to the analysis by BlotCyclerTM using the same antibodies as described in Fig. 2. The results on Fig. 3 demonstrated excellent reproducibility as seen visually (Fig. 3A). To confirm this quantitatively, the band intensity for each protein was quantified and normalized against the marker. The calculations shown in Fig. 3B are consistent with visual observations and suggest that the salient characteristics of BlotCyclerTM, such as timing and consistency of solution changes, efficient washing, and elimination of operator caused errors, would enable the much-needed standardization of western blotting.
Fig. 3A Fig. 3B
 Fig. 3. Automatic western blot processing by BlotCyclerTM yielded highly reproducible results. (A) Images of the five identical blots where the first lane contained a HeLa whole cell lysate, the second lane contained a recombinant Human IL-2, and the third lane had a mixture of HeLa whole cell lysate and h-IL2. After SDS-PAGE electrophoresis, proteins were transferred from the gel onto the Nitrocellulose membrane. Membranes were blocked for 90 min using MB-070. and then incubated with the rabbit anti-IL2 and mouse anti-α-tubulin primary antibodies for 18 hours, followed by the incubation with DyLight™ 549 conjugated anti-mouse IgG and DyLight™ 649 conjugated anti-rabbit IgG secondary antibodies for 90 min. All incubation steps were performed at 40C. Data for each DyLight™ fluorophore was collected independently at excitation/emission wavelengths: 530/605 nm for the DyLight™ 549 and 625/695 nm for DyLight™ 649. The calculated fluorescent intensity between different blots was normalized using MW standard as shown in (B).
Fig. 3. Automatic western blot processing by BlotCyclerTM yielded highly reproducible results. (A) Images of the five identical blots where the first lane contained a HeLa whole cell lysate, the second lane contained a recombinant Human IL-2, and the third lane had a mixture of HeLa whole cell lysate and h-IL2. After SDS-PAGE electrophoresis, proteins were transferred from the gel onto the Nitrocellulose membrane. Membranes were blocked for 90 min using MB-070. and then incubated with the rabbit anti-IL2 and mouse anti-α-tubulin primary antibodies for 18 hours, followed by the incubation with DyLight™ 549 conjugated anti-mouse IgG and DyLight™ 649 conjugated anti-rabbit IgG secondary antibodies for 90 min. All incubation steps were performed at 40C. Data for each DyLight™ fluorophore was collected independently at excitation/emission wavelengths: 530/605 nm for the DyLight™ 549 and 625/695 nm for DyLight™ 649. The calculated fluorescent intensity between different blots was normalized using MW standard as shown in (B).
Throughput and reproducibility of BlotCyclerTM enabled rigorous optimization of the antibody concentration
Optimal concentrations of both primary and secondary antibodies against a given antigen are essential to obtain the most informative results and are highly dependent on their specific activities. BlotCyclerTMallows simultaneous processing of up to six different blots with six different secondary or primary antibodies at the same time with high reproducibility, thereby avoiding the inherent slight variations associated with the manual processing of multiple blots at the same time. An example of such optimization is shown in Fig. 4. Here, ten identical strips that contained MW marker and mouse pancreas extract were prepared and probed with five different dilutions of primary antibody against Delta-4 (Fig. 4A) and a single dilution of secondary antibody. The second experiment contained a single dilution of primary antibody) and six different dilutions of the secondary antibody (Fig. 4B). Using BlotCycler™ we were able to construct the titration curves for both primary and secondary antibodies.
Fig. 4 A

BlotCyclerTM enabled optimization of antibody concentrations.
(A) Titration of the primary rabbit anti-Delta-4 antibody with a fixed secondary DyLight™ 649 conjugated anti-rabbit IgG antibody concentration (1:20,000). (B) Titration of the secondary antibody with a fixed primary antibody concentration (1:1,000). The gel was loaded with an extract of mouse pancreas in all lanes. Electrophoresis was run as described. Proteins were transferred from the gel onto the Nitrocellulose membrane. Membranes were blocked for 90 min using MB-070, and then incubated with the primary antibody for 18 hours, followed by the incubation with the secondary antibody for 90 min. All incubation steps were performed at 4°C. Data was collected independently using band pass filter at 625/695 nm for DyLight™ 649. The fluorescent intensity between different blots was normalized using MW standard.